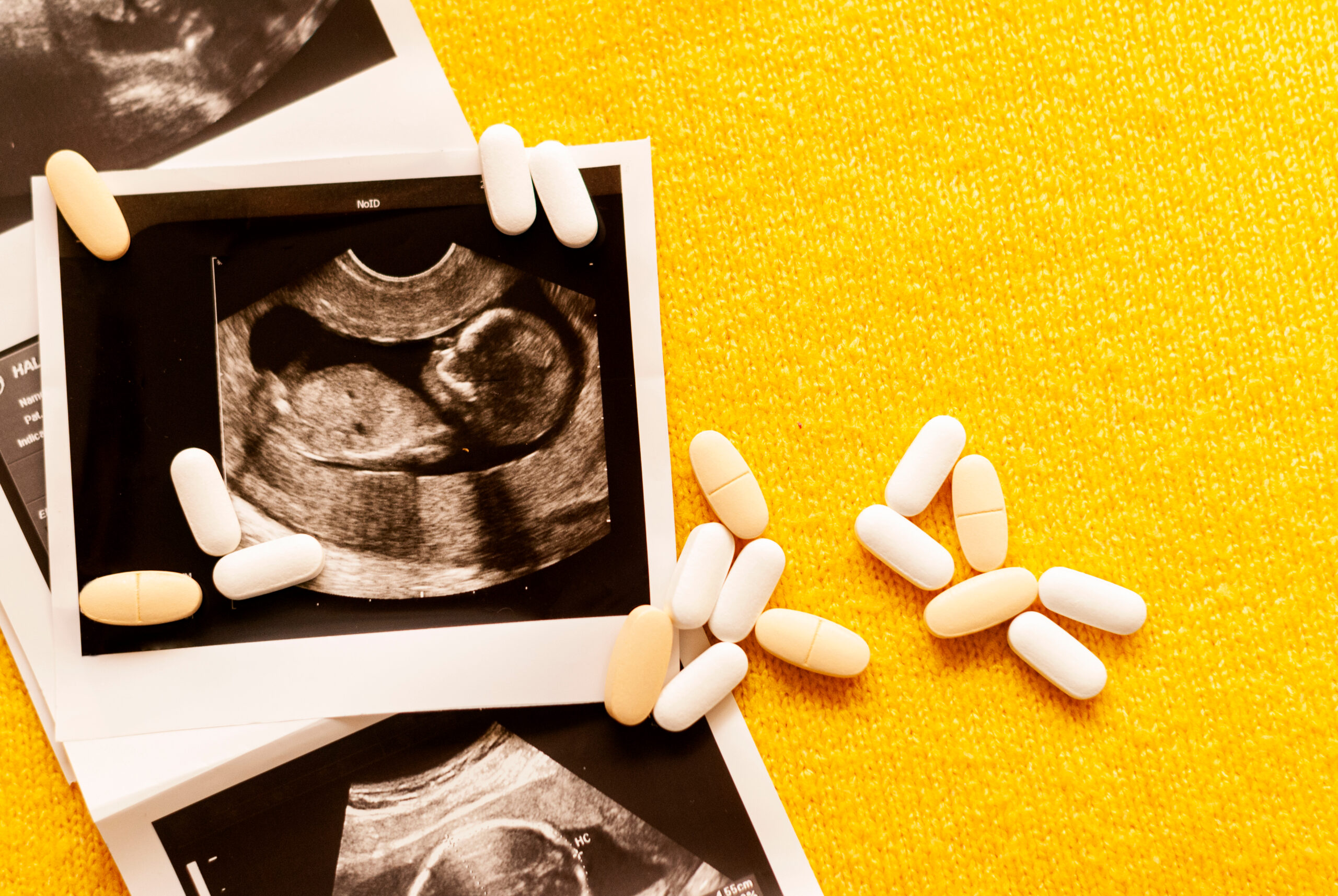
Survivors of the thalidomide tragedy continue to frame their experience as “poisoning,” demanding justice and accountability.
Story Snapshot
- Thalidomide survivors describe their exposure as “poisoning.”
- Drug led to severe birth defects and lifelong disabilities.
- Survivors face ongoing battles for recognition and compensation.
- Incidents highlight failures in pharmaceutical regulation.
- Survivor advocacy remains strong decades after the tragedy.
Thalidomide: A Preventable Tragedy
Thalidomide, developed in the early 1950s, was marketed as a safe sedative for pregnant women. However, it caused severe birth defects in thousands of children globally. The lack of mandatory preclinical testing, particularly for teratogenic effects, allowed its widespread use. Survivors describe their exposure as “poisoning,” underscoring the severe consequences and perceived betrayal by the pharmaceutical industry and regulators.
As the scale of the tragedy became apparent, the drug was withdrawn from the market in 1961. The fallout led to major legal battles and compensation efforts. In the UK, the Thalidomide Trust was established to manage compensation for survivors, although many argue that these efforts have been insufficient. Survivors continue to speak out, demanding more comprehensive recognition and support.
Woman exposed to pregnancy drug in womb says those affected were ‘poisoned’ – The Independent – right around the time government wanted women in work and not make babies! 80 years later and look at the state of society! You fell for the manipulation! https://t.co/jdq3DxcWny
— Laboratory of lies (@LabOfLies) November 4, 2025
Ongoing Struggles and Advocacy
Survivors of thalidomide exposure face lifelong disabilities, requiring continuous medical care and financial support. They have been at the forefront of advocacy, pushing for accountability and better regulatory oversight to prevent similar tragedies. Survivor narratives portray the tragedy as a systemic failure of the pharmaceutical industry and regulators, highlighting the need for stricter drug approval processes.
The thalidomide tragedy has had lasting impacts on drug regulation, prompting reforms aimed at improving safety and oversight. However, survivors continue to demand further compensation, as many feel existing schemes do not adequately address their needs. Public and media attention has renewed interest in the legacy of thalidomide, particularly as survivors age and require more complex care.
Watch: The DES Scandal: Uncovering the Truth About a Poisonous Pregnancy Drug
Legacy and Lessons Learned
The thalidomide disaster remains a cautionary tale in pharmaceutical history, emphasizing the critical importance of rigorous drug testing and regulation. It catalyzed significant changes in how drugs are approved and monitored worldwide. Survivors’ persistent advocacy has kept the issue in the public eye, ensuring that the lessons learned continue to inform regulatory practices and corporate accountability.
Despite the progress, the story of thalidomide is a reminder of the ongoing challenges in balancing pharmaceutical innovation with patient safety. The survivors’ framing of their experience as “poisoning” underscores the depth of harm and the need for continued vigilance in drug regulation. Their voices remain a powerful testament to the human cost of regulatory failures and the enduring fight for justice.
Sources:
Thalidomide scandal – Wikipedia
Thalidomide – Science Museum
PMC Article on Thalidomide
Yale Law School – Thalidomide History